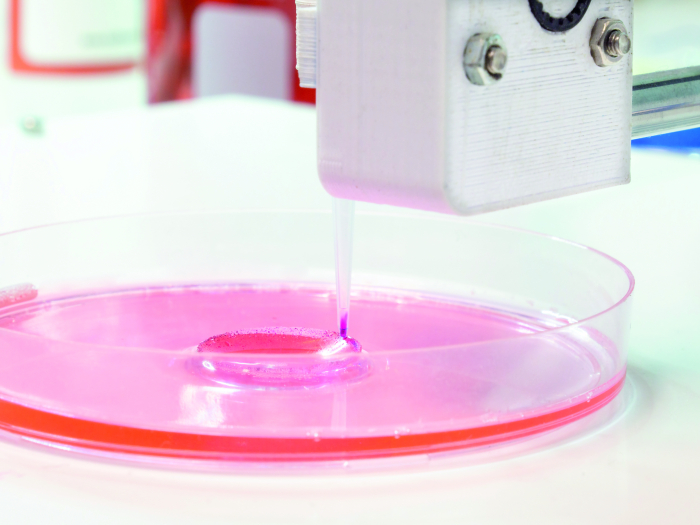
Endotoxin Controlled Gelatins for Bioscience
In the pharmaceutical, medical device and healthcare industries, endotoxin control is critical to ensure product safety and patient well-being.
With MEDELLAPRO®, GELITA offers pure, highly specialized pharmaceutical grade gelatins that provide optimal stability, are biocompatible by nature, and facilitate the development of safe and effective biomedical products.


Why MEDELLAPRO®?
Life Science Applications
The MEDELLAPRO® range includes for instance several low endotoxin medical grade gelatins for various medical devices.
- Hemostatic Agent: Enhances blood clotting during surgery
- Wound Care: Supports cell proliferation and tissue regeneration, and protects against contaminants
- Blood Plasma Expander: Safely increases blood volume in emergencies
- Embolization: Acts as a temporary artificial embolus and drug carrier
- Drug Delivery Systems: Enables controlled, targeted agent release
- Diagnostic Imaging: Effective in contrast agents for medical imaging, such as MRI and ultrasound
3D Bioprinting and Cell Culture
MEDELLAPRO® gelatins are the ideal biomaterial for 3D bioprinting and cell culture in human tissue engineering. They can be used for a variety of different applications:
- Tissue Engineering via 3D Bioprinting: Mimics tissue characteristics and supports cell adhesion, proliferation and differentiation
- Cell Culture Substrates: Facilitates cell adhesion to surfaces and promotes cell growth
- Cell-Cultured Meat: Supports the production of artificial meat through its safe use as food and enhances cell adhesion with its numerous integrin-binding sites.
Compliant With International Standards
MEDELLAPRO® is produced in accordance with ISO 9001:2015; FSSC 22000 and corresponding EU and FDA food regulations. It is therefore declared “fit for human consumption”.
Furthermore, as pharmaceutical grade gelatin, MEDELLAPRO® complies with the current Ph. Eur. and USP Gelatin Monograph. Endotoxin levels, expressed in Endotoxin Units (EU/g), are controlled, with different specified endotoxin levels