By preventing troublesome crosslinking, breakthrough gelatin technology is enabling pharmaceutical and supplement manufacturers to guarantee the stability of softgels destined for the Asia-Pacific market.
Softgel capsules are set for rapid growth in the next five years, with Asia-Pacific leading the way. The region’s softgel market is projected to expand by 6.6% year on year between now and 2027, and for countries such as India and China, even stronger growth is forecast. [1]
Softgels offer several advantages that are driving their adoption. They are hermetically sealed to make them airtight. This not only helps them to protect sensitive ingredients, but also makes them an easy-to-swallow delivery format for unpalatable fills. They also offer higher dosing accuracy than other formats.
However, even with all these factors in their favour, there is one major problem that threatens to hamper the growth of softgels in Asia-Pacific: the impact of heat and humidity on product stability.
Climatic Considerations
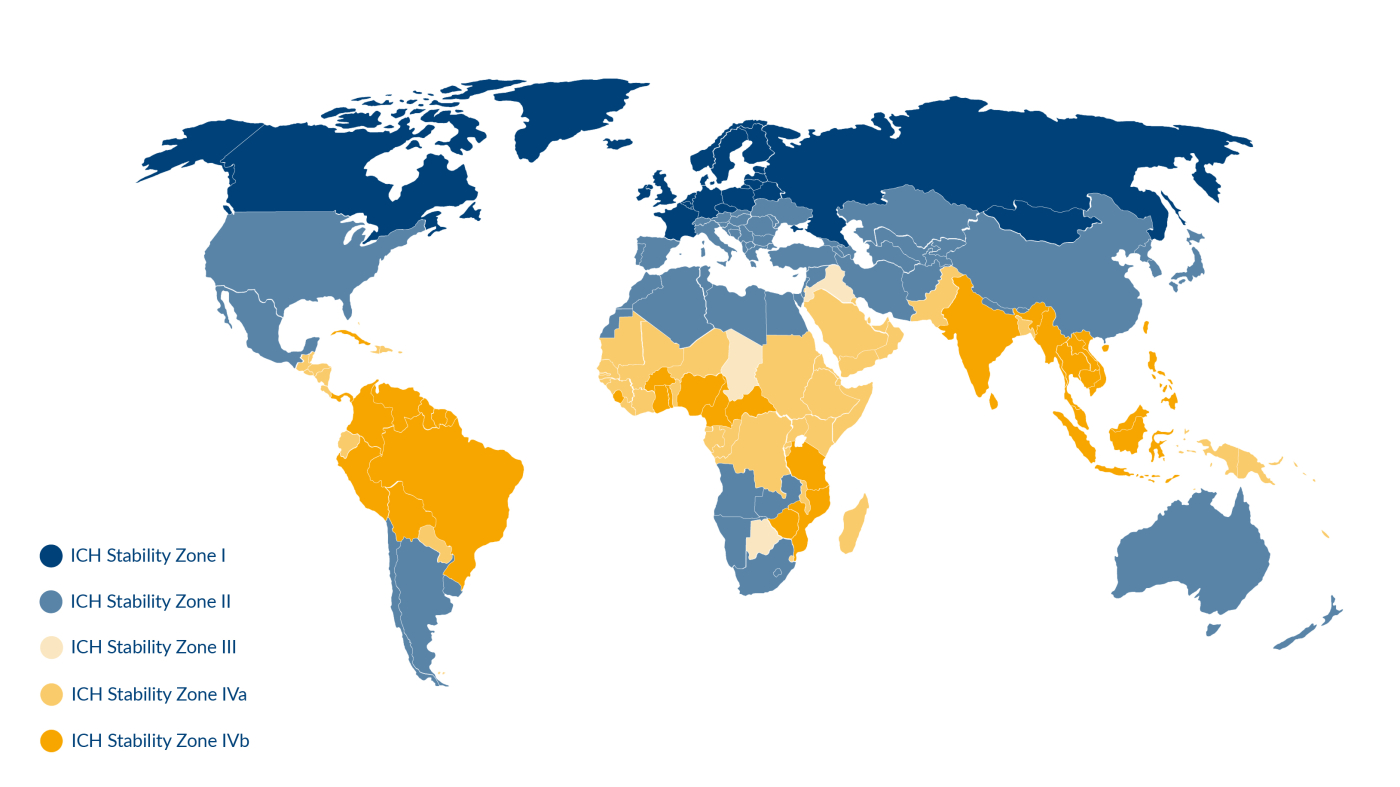
According to the ICH guidelines for stability studies, the world is divided into five climatic zones (I, II, III, IVa and IVb). Countries with temperate climates fall into zone I, whilst, at the other end of the scale, countries with very hot and humid climates (most Asia-Pacific countries) are classed as zone IVb. Exposure to these extreme climatic conditions can have a detrimental effect on softgel stability and shelf life.
A Meeting of Molecules
Heat and moisture provide the optimal conditions for crosslinking of the gelatin shell. Crosslinking occurs when protein molecules in the shell interact with compounds containing reactive molecules such as aldehydes, ketones, terpenes and peroxide intermediates. These substances are often found in fruity and herbal flavourings and extracts. They can also occur as a result of oxidation and/or the presence of metals such as iron, which are used as shell pigments.
Over time, crosslinking causes capsule solubility to decline, which results in longer dissolution times in the GI (gastro intestinal) tract and slower fill release rates.
Blocking Interaction
The pharmaceutical industry has developed additives that reduce crosslinking with varying degrees of success. We have taken a different approach to addressing this problem, developing a gelatin grade that essentially protects itself against crosslinking.
We call this patented technology RXL®® Portfolio (reduced crosslinking) as deactivates the gelatin’s ability to interact with reactive molecules. For companies operating in the Asia-Pacific region, this is a game-changer as it extends shelf life and guarantees reliable fill release, even in hot and humid conditions.
The Power of Three
There are three pharmaceutical grade gelatins in the RXL®® Portfolio: RXL®® Prime and RXL®® Advanced ensure the long-term capsule stability for critical fills. There is also RAPISOL®® for applications in which faster release is key. This could include painkillers or cough and cold formulas - products where there is an expectation that they will deliver rapid relief. Hot and humid storage conditions delay the stated effect by slowing down fill release, which is where RAPISOL®® can make a difference.
A further advantage of using RXL®® in soft gels is that it offers a direct route to a stable formulation - manufacturers waste less time fine-tuning formulations to meet stability testing requirements during the development phase, which translates to shorter time to market.
Harnessing a hot Opportunity
The Asia-Pacific market offers attractive potential for softgels, but the climatic conditions can be a barrier to entry. By countering the problem of crosslinking, the RXL®® Portfolio can provide the capsule performance boost that manufacturers need to scale that hurdle.
If you want to find out more about formulating softgels with our RXL®® Portfolio, please contact our team of experts today.
[1] Pharmaceutical Gelatin Market, Global Forecast to 2027, MarketsandMarkets