Enteric Capsules with
GELITA® EC Gelatin Product
Expanding GELITA’s Portfolio of Capsule Release Possibilities
GELITA offers a comprehensive portfolio of gelatins that provides a broad release profile in soft capsules. Besides a variety of versatile and tailor-made standard gelatins, there are GELITA® RXL and GELITA® RXL Advanced for improved capsule shelf-life and stability. These special gelatins provide reliable release and reduced cross-linking properties, even under extreme storage conditions. This GELITA capsule gelatin portfolio is complemented by GELITA® RXL R² for revolutionary fill release. The soft capsules produced with GELITA® RXL R² offer a faster release of the fill and offer the added advantage of the reduced cross-linking properties of the RXL technology.
And, now – on the opposite end of the release profile spectrum, GELITA offers GELITA® EC; a gelatin product for the one-step production of truly enteric capsules.

Satisfying Consumer Demand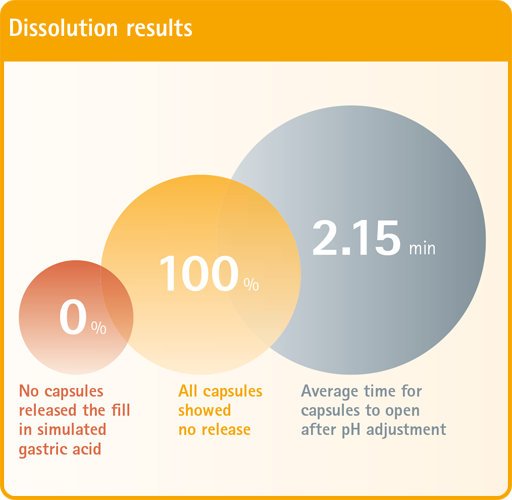 Consumers have been ingesting nutrients in soft capsules for decades due to their ease of swallowing and convenience. However, one nagging issue has been plaguing consumers for as long as they’ve been ingesting fish oil and other omega-3 fatty acids: fishy burps and aftertastes. This undesirable effect has even demotivated the otherwise health-conscious consumers to avoid taking fish oil supplements altogether. Until now. GELITA® EC – the first commercially available gelatine product of its kind – allows for the one-step production of enteric capsules that open in the intestine (instead of the stomach, as with traditional gelatine capsules). |
Added Value with GELITA® EC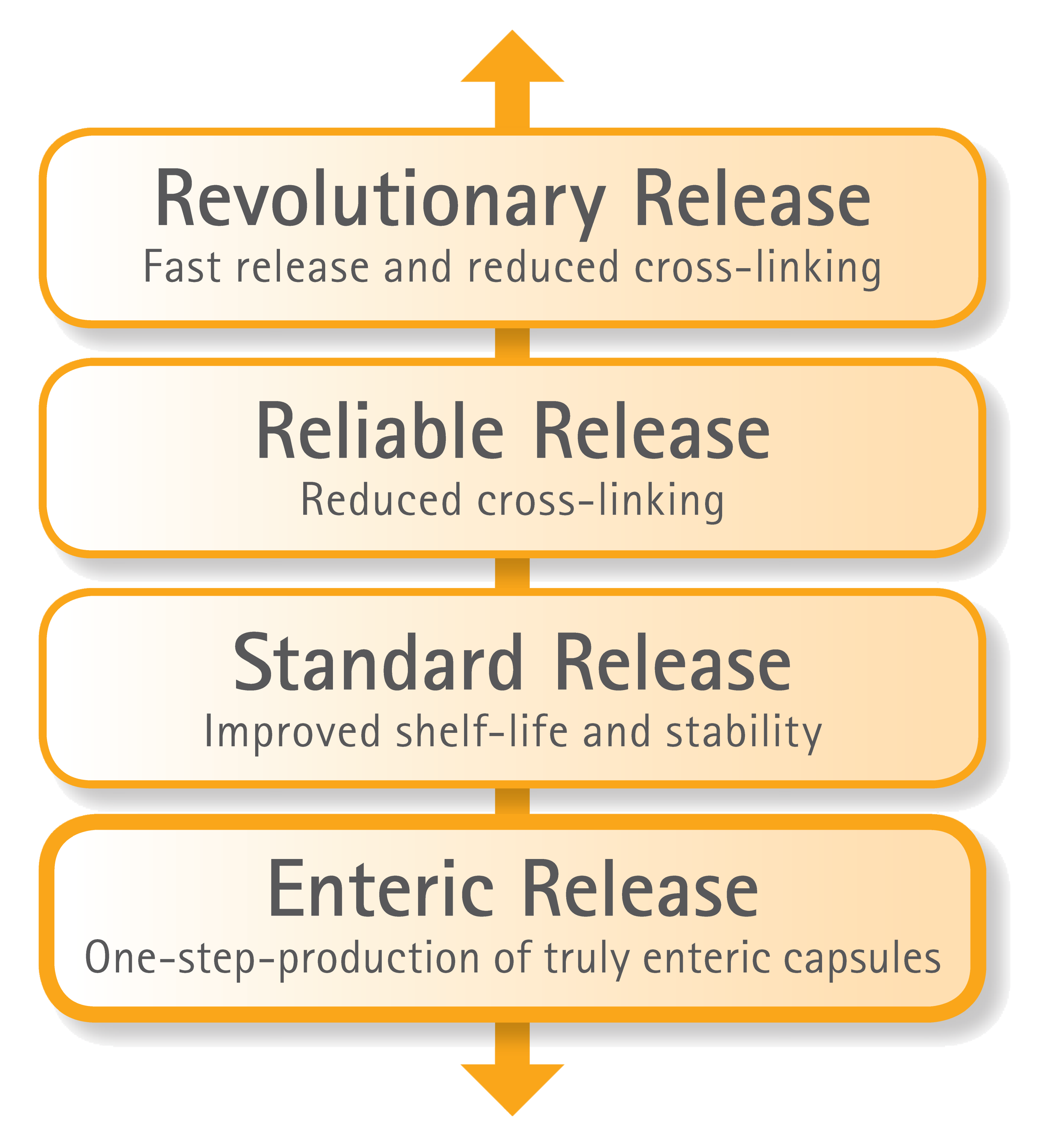 Most enteric delivery systems are produced by applying an acid-insoluble coating to freshly produced soft capsules. This intensive two-step process adds time and money to the cost of each enteric capsule. Additionally, this coating produces an opaque shell – which is less desired by consumers. With GELITA® EC, capsules producers now have the ability to create enteric capsules using existing equipment in a one-step process – avoiding additional time and costs – while creating brilliantly clear capsules. |
Proven Enteric Release GELITA® EC allows manufacturers to produce enteric capsules that adhere to USP (US Pharmacopeia) dissolution parameters. These parameters include:
Until now. GELITA® EC – the first commercially available gelatine product of its kind – allows for the one-step production of enteric capsules that open in the intestine (instead of the stomach, as with traditional gelatine capsules).
|
GELITA® EC Add Value to Consumers and Industry Most enteric delivery systems are produced by applying an acid-insoluble coating to freshly produced soft capsules. This intensive two-step process adds time and money to the cost of each enteric capsule. Additionally, this coating produces an opaque shell – which is less desired by consumers. Benefits of GELITA® EC
|



